The Department of Health and Human Services under Secretary Robert F. Kennedy Jr. has emerged as the most aggressive federal agency in advancing President Donald Trump’s second-term agenda, according to federal regulatory data reviewed by The Dallas Express.
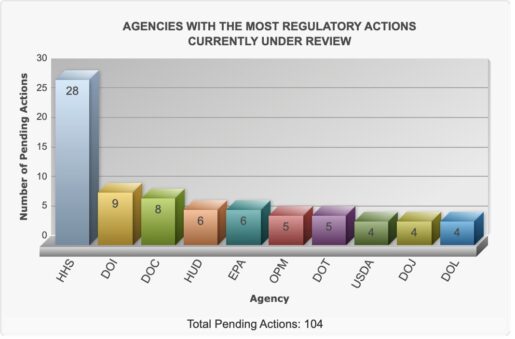
HHS currently has 28 regulations under review, far outpacing all other Cabinet departments. The next most active agencies, the Interior Department and the Commerce Department, have just nine and eight rules under review, respectively.
Although the Department of Homeland Security arguably draws the most headlines in Trump-era policy debates, it trails with only two, according to the regulatory tracker on regulations.gov.
Many of the HHS measures reflect Trump’s “Make America Healthy Again” (MAHA) initiative — a core part of his 2024 campaign platform after Kennedy joined the coalition supporting the Republican ticket following the party’s 2024 national convention. The MAHA movement has generally sought to realign public health policy around domestic drug production, medical transparency, and restrictions on taxpayer funding for controversial procedures on minors, among other policy goals.
Among the most closely watched proposals under review are:
- The “GLOBE” Model (Global Benchmark for Efficient Drug Pricing) — aimed at pressuring pharmaceutical manufacturers to match U.S. drug prices to international benchmarks.
- The “GUARD” Model (Guarding U.S. Medicare Against Rising Drug Costs) — designed to limit price escalation across Medicare programs.
- A proposed rule prohibiting federal Medicaid funding for “sex trait modification procedures” for minors, part of a broader HHS initiative to limit what it defines as “non-essential medical interventions” on children.
The rule prohibiting taxpayer funding for “transgender” treatments will reportedly apply to hospitals that provide puberty blockers, cross-sex hormones, and “gender-transition “surgeries to children with money from the Children’s Health Insurance Program, National Review exclusively reported.
The details of the GLOBE Model have not yet been made public. However, many suspect it will resemble a 2020 Trump rule tying what Medicare pays for 50 drugs to the lowest prices in select countries, per Reuters. The initial iteration of this rule was blocked in the courts.
Almost nothing is publicly known about the GUARD Model beyond the implication that HHS leadership is considering a cap to limit periodic increases in drug prices. “OMB review is the last stage in the federal government’s internal clearance process before a rule is issued. However, given the government shutdown, it is unclear when the rules will be issued,” Jeffrey Davis,
Other pending HHS rules would repeal Biden-era staffing mandates for long-term care facilities, expand Medicare’s domestic procurement of protective equipment and essential medicines, and restore flexibility to child care block grants.
Taken together, the department’s 28 pending regulatory actions, spanning Medicare, Medicaid, the FDA, and child welfare programs, suggest Kennedy’s HHS has become the nerve center of the Trump administration’s policy implementation effort.
By comparison, the Interior Department’s nine pending rules focus mainly on oil, gas, and endangered species management, while Commerce’s eight cover export controls and communications technology. More than 100 pending regulatory actions across agencies, according to the Office of Information and Regulatory Affairs.