The U.S. Food and Drug Administration has recommended halting the use of phenylephrine in its oral form, a widely used nasal decongestant.
This decision is primarily driven by questions regarding the drug’s effectiveness rather than any safety issues, reported Fox 4 KDFW.
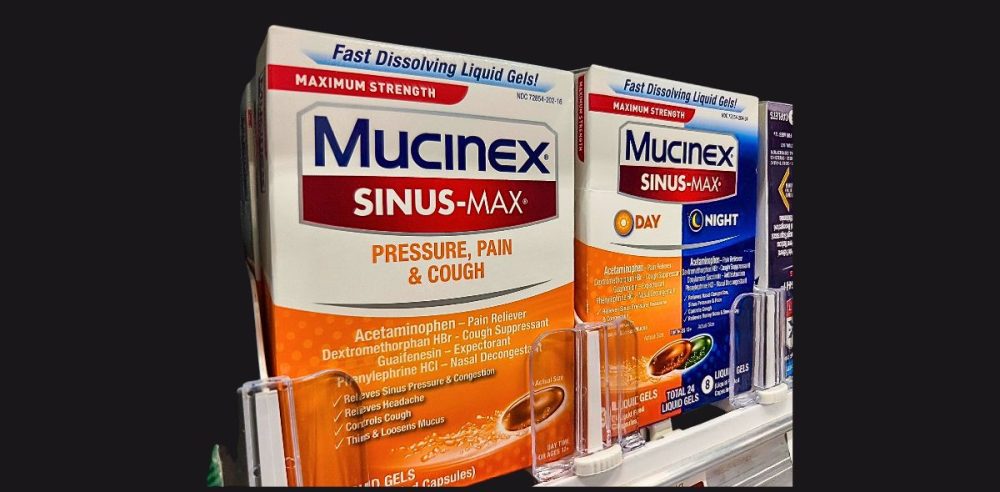
Phenylephrine is a common component in a variety of over-the-counter pills and liquid medications that relieve nasal congestion.
As the FDA moves forward with this proposal, it raises important considerations for consumers relying on these medications and cold and allergy relief product manufacturers.
Here is more of what Fox 4 reported on the changes in how nasal decongestants may be produced and marketed in the future:
The U.S. Food and Drug Administration this month announced its proposal to remove a popular decongestant found in many over-the-counter medications from shelves.
The decongestant in question is phenylephrine, which is found in popular cough and cold products, including Benadryl Allergy Plus Congestion, Sudafed PE Sinus Congestion and Vick’s DayQuil.
The reason for the FDA’s proposal isn’t due to safety, but, rather, over the ongoing concern that the drug isn’t effective when taken in oral form.
Here is what to know about it:
What is phenylephrine?
Phenylephrine has been used for temporary relief of nasal congestion since being approved by federal health regulators in 1976.
By 2006, it became the main ingredient in many over-the-counter medicines, replacing the older ingredient pseudoephedrine, which was moved behind counters because it could be processed into methamphetamine.
Some products contain only oral phenylephrine as a single, active ingredient, while others contain oral phenylephrine and another active ingredient, such as acetaminophen or dextromethorphan.
“The presence of oral phenylephrine in these medicines does not affect how other active ingredients work to treat the symptoms for which they are intended,” the FDA noted.
Why is phenylephrine under review?
The effectiveness of phenylephrine when taken as a pill or liquid has been questioned for some time.
Studies conducted in the past few years by the drugmakers Merck and Johnson & Johnson have shown no difference between phenylephrine medications and placebos for relieving congestion, according to The Associated Press.
As such, last year, CVS Health pulled from its shelves oral decongestants that contained phenylephrine as the only active ingredient.
“Think of it almost like a useless drug that’s sitting in there,” Dr. Dipak Chandy, who is with New York’s Westchester Medical Center, told FOX Business in 2023 when CVS was removing the products. “It’s doing nothing, and it’s doing no harm. It’s doing no good. … It’s really a completely pointless drug that’s mixed in with a bunch of other medications.”
The FDA said the drug appears more effective when applied directly to the nose, in sprays or drops. Those products are not under review.
The Consumer Healthcare Products Association said last week it was disappointed in the FDA’s latest proposal to remove oral phenylephrine from shelves.
“We believe Americans deserve access to safe and effective OTC medicines and the option to choose the products they prefer for self-care,” their statement read, in part.