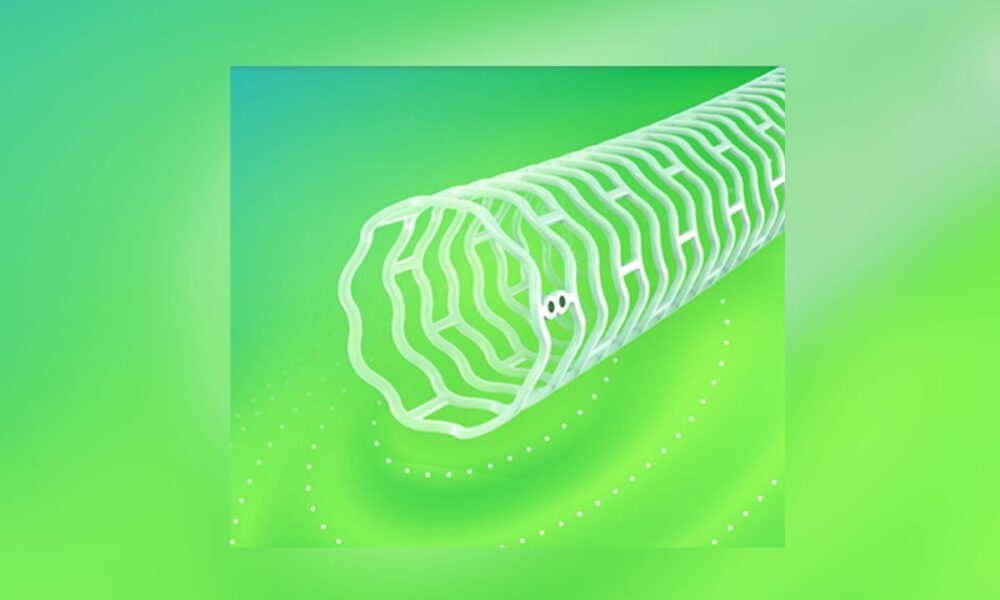
Abbott announced Monday it has received European regulatory approval for a revolutionary dissolving stent designed to treat severe peripheral artery disease (PAD) in the legs.
Abbott is a worldwide leader in healthcare dedicated to helping individuals live their lives to the fullest, regardless of their age or stage in life.
The groundbreaking device marks the first of its kind for treating blockages below the knee. The approval matters for 50 million Europeans living with peripheral artery disease, a condition that blocks blood flow to the lower extremities. Until now, patients faced limited treatment options and potential amputations.
The Esprit BTK Everolimus Eluting Resorbable Scaffold System uses material similar to dissolving sutures. After opening blocked arteries and restoring blood flow, the stent gradually disappears, leaving no permanent implant behind.
“For too long, patients with severe PAD below the knee have had limited treatment options and were often faced with potential amputations,” said Professor Dierk Scheinert, M.D., of University Hospital Leipzig. “With CE Mark, the Esprit BTK System offers a resorbable scaffold backed by strong data and proven superiority over balloon angioplasty — giving physicians a novel, innovative tool to treat the most severe forms of PAD more effectively and improve patient outcomes across Europe.”
The device represents a significant advance over balloon angioplasty, the current standard treatment. That procedure often fails when vessels become blocked again, requiring additional interventions.
Clinical data from the LIFE-BTK trial demonstrated 48% fewer repeat procedures compared to balloon angioplasty over two years. The trial results were presented at the Vascular InterVentional Advances Conference in November 2024.
Peripheral artery disease occurs when plaque clogs arteries, preventing blood and oxygen from reaching the feet and lower legs. The condition causes severe pain, non-healing wounds, and sometimes requires limb amputation.
Over ten years, the most severe PAD cases show only 25% survival rates. Despite affecting roughly twice as many Europeans as Americans, public awareness remains low.
“Abbott is an expert in the development of innovative treatments for vascular diseases, and pioneered dissolving stents for people with PAD below the knee,” said Samih Al Mawass, Abbott’s divisional vice president for Europe, Middle East & Africa. “With the Esprit BTK System, we’re helping to restore blood flow without leaving a permanent implant behind.”
The minimally invasive procedure involves inserting the scaffold through a small leg incision using a catheter. The device delivers everolimus medication to support vessel healing before dissolving completely.
The U.S. Food and Drug Administration approved the Esprit BTK System in April 2024. Abbott, based in Illinois, employs 114,000 people and operates in more than 160 countries.