A groundbreaking biodegradable heart patch, developed by researchers at ETH Zurich and the University Hospital of Zurich, promises to repair damaged heart muscle by merging with tissue and dissolving after healing.
Unveiled in a study published in Advanced Materials, the reinforced cardiac tissue patch, dubbed RCPatch, could transform treatment for heart attack survivors.
Heart attacks starve heart tissue of blood and oxygen, often damaging muscle and, in severe cases, risking rupture of the heart wall. Surgeons typically use patches made from bovine pericardium — a membrane from cow hearts — valued for stability and ease of use. However, these remain as foreign objects, potentially causing inflammation, calcification, or clots over time.
“Traditional heart patches do not integrate into the heart tissue and remain permanently in the body,” said Lewis Jones, the study’s lead author, per Brighter Side. “We wanted to solve this problem with our patch, which integrates into the existing heart tissue.”
Led by Professors Robert Katzschmann and Omer Dzemali, the team designed the RCPatch to go beyond sealing defects.
“Our goal was to develop a patch that not only closes a defect but also helps to repair it completely,” Katzschmann said, Brighter Side reported.
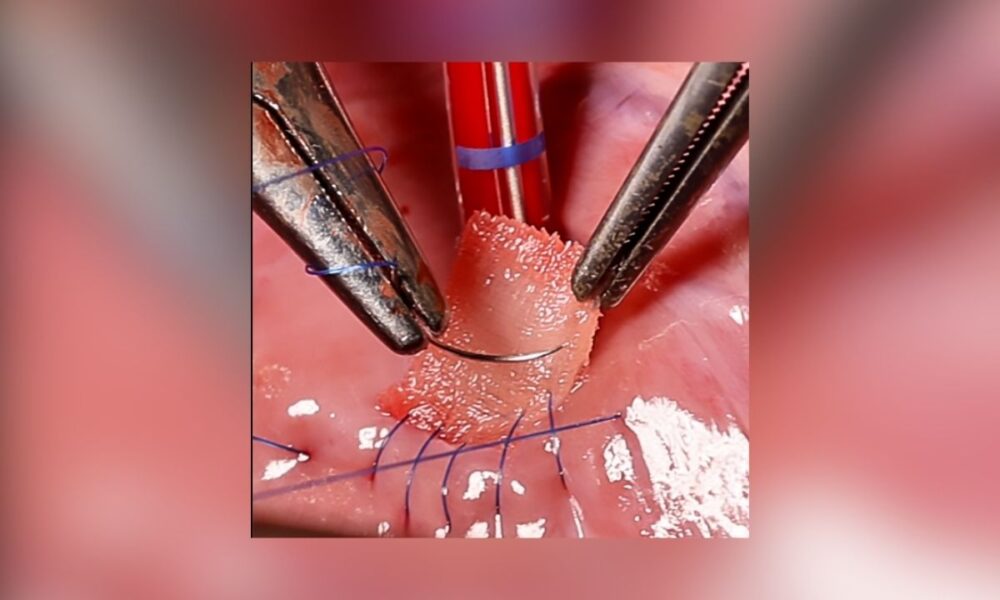
The patch combines a fine mesh, a 3D-printed scaffold of biodegradable polycaprolactone, and a hydrogel infused with living heart muscle cells.
The lattice-patterned scaffold, crafted via high-precision volumetric printing, provides strength, while the hydrogel encourages tissue integration. A suturable mesh, also soaked in hydrogel, anchors the patch to the heart wall, allowing it to degrade as cells merge with the myocardium.
Engineered for the heart’s high-pressure environment, the patch balances strength and flexibility.
The team used melt-electrowriting to create a less permeable mesh soaked in fibrin hydrogel, reducing blood leakage risks. Its anisotropic design adjusts stiffness to match the heart’s complex motion, optimized through computational models to withstand pressures like the 81 mmHg recorded before surgery in pig trials, dropping to 66 mmHg post-implantation without bleeding.
Initial tests on pig hearts with artificial left ventricle defects showed the patch maintained integrity and restored stable function. Future studies will assess long-term integration and degradation, unlike current patches — whether bovine, polytetrafluoroethylene, or polyester — which offer no regeneration and remain lifelong. The RCPatch aims to leave only repaired tissue, addressing complications, especially in younger patients.
Katzschmann envisions a future where such patches “don’t just close a defect but help the heart regenerate. That’s the real goal,” Brighter Side reported.
Still in preclinical stages, the innovation could shift cardiac surgery if human trials succeed, offering patients fewer complications and a patch that becomes part of their own heart.