Dilbert creator Scott Adams’ plea for help in accessing a life-prolonging cancer treatment drew rapid responses from President Donald Trump and Health and Human Services Secretary Robert F. Kennedy Jr. over the weekend.
Adams, who said he is suffering from metastasized prostate cancer, posted early Sunday that he was unable to schedule his first dose of Pluvicto, a recently approved radioligand therapy used to treat advanced prostate cancer.
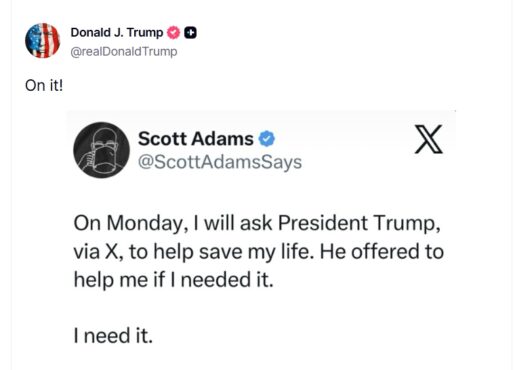
At 3:29 a.m. on November 2, Adams wrote on X, “On Monday, I will ask President Trump, via X, to help save my life. He offered to help me if I needed it. I need it. As many of you know, I have metastasized prostate cancer. My healthcare provider, Kaiser of Northern California, has approved my application to receive a newly FDA-approved drug called Pluvicto. But they have dropped the ball in scheduling the brief IV to administer it and I can’t seem to fix that. I am declining fast. I will ask President Trump if he can get Kaiser of Northern California to respond and schedule it for Monday. That will give me a fighting chance to stick around on this planet a little bit longer. It is not a cure, but it does give good results to many people.”
Trump replied within hours with a short message on Truth Social: “On it.”
Kennedy, the sitting HHS Secretary, reportedly followed up on X, writing, “Scott. How do I reach you? The President wants to help,” at 10:09 a.m. on November 2.
They apparently worked fast, according to Adams’ November 3 post:
Update: Getting Pluvicto (the cancer drug) tomorrow, via Kaiser Northern California.
The Trump administration works fast.
Amazing.
For context, I waited months for the drug, like everyone else. But I think my files got misplaced or something and that glitch just got…
— Scott Adams (@ScottAdamsSays) November 3, 2025
Pluvicto, developed by Novartis, was approved by the U.S. Food and Drug Administration in 2022 for adults with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer who have undergone prior hormone and chemotherapy treatments.
The FDA press release said the drug demonstrated “a statistically significant improvement in the primary endpoints of overall survival and radiographic progression-free survival.”
Clinical trials cited by the agency found a median overall survival of 15.3 months for patients receiving Pluvicto compared to 11.3 months for those on the best standard of care. Reported side effects included fatigue, dry mouth, anemia, nausea, and myelosuppression.
Dr. Michael Hofman, an Australian nuclear medicine physician, estimated in 2022 that each Pluvicto treatment cycle costs more than $40,000 in the United States, with a typical course involving six cycles. However, Pluvicto’s manufacturer website says it can help patients with private insurance “pay as little as $0” through its Co-Pay Plus program.
The Moffitt Cancer Center website notes that “while Pluvicto is not a cure for prostate cancer, clinical trials have shown that it can help slow cancer progression, reduce the tumor burden and improve the outcome and quality of life for patients with advanced prostate cancer.”
Men diagnosed with distant-stage prostate cancer have a five-year relative survival rate of about 37%, compared to over 99% for localized cases, according to the American Cancer Society.
Adams’ plea highlights the ongoing challenges some cancer patients face in obtaining newly approved therapies.
A spokesman for Kaiser Permanente told USA Today, “Mr. Adams’ oncology team is working closely with him on the next steps in his cancer care, which are already underway. Since it was approved by the FDA three years ago, Kaiser Permanente’s nuclear medicine and medical oncology experts have treated more than 150 patients with Lu-177 PSMA (Pluvicto) in Northern California alone. We know this drug and this disease.”
Trump, who championed the federal “Right to Try” law in 2018 to expand patient access to experimental drugs, has frequently cited that legislation as part of his administration’s broader push to accelerate access to life-saving treatments for the seriously ill.
The Right to Try Act allows terminally ill patients to seek investigational drugs that have completed Phase 1 clinical trials without FDA approval. Though critics, including the advocacy group FORCE, warned in a press release that it could “be detrimental to patients” and limit oversight intended to protect patient safety.