Surviving victims of an IV-tampering scheme by a disgraced former doctor are suing the manufacturer of the product for its failure to provide adequate safeguards against tampering.
In September 2022, The Dallas Express first broke the story on the link between Dr. Raynaldo Riviera Ortiz Jr., an anesthesiologist, and the discovery of tainted IV bags at Baylor Scott & White Surgicare Center in Garland, where he worked.
Between May and August of that year, numerous patients at the surgical center experienced unexplained life-threatening cardiac emergencies. Melanie Kaspar, an anesthesiologist at the facility who took home an IV bag from the surgical center to treat herself for dehydration, died from a heart attack.
Shortly after Kaspar’s death, an 18-year-old patient undergoing a routine sinus surgery at the facility was rushed to the ICU in critical condition. Doctors reviewing the records noticed that the patient’s adverse medical reaction began shortly after a new IV bag was hung, and they began to suspect that the IV bag was tainted.
A lab analysis revealed that the saline solution in the bag was contaminated with bupivacaine, a nerve-blocking agent; epinephrine, a stimulant; and lidocaine, an anesthetic. The combination of drugs was likely the cause of the teen’s symptoms, which included very high blood pressure, cardiac dysfunction, and pulmonary edema.
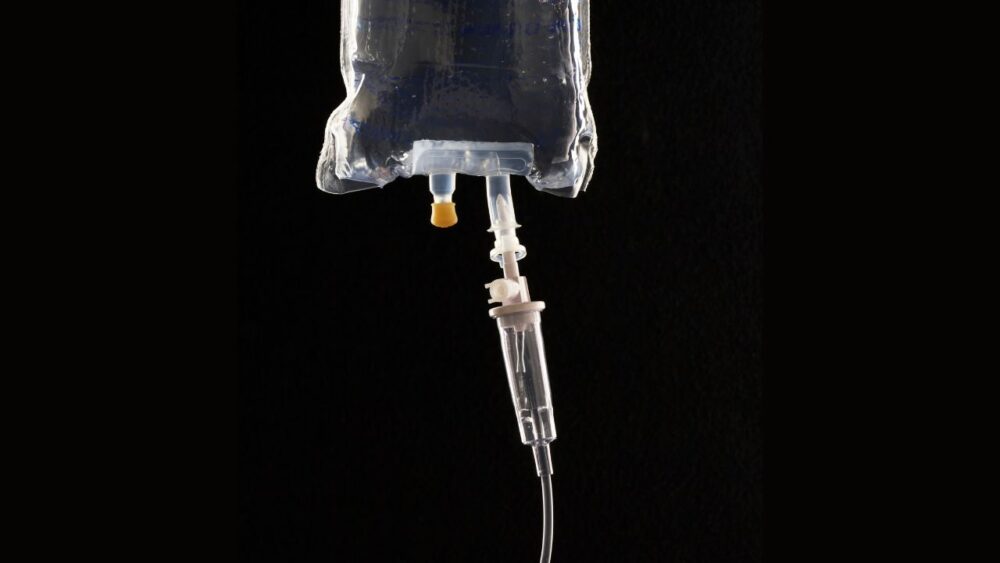
Lab analysts also observed a puncture in the plastic casing surrounding the IV bag.
The IV bags, manufactured by Baxter International, came with a safety cap on one port, but none was provided for the second port on the bag, which could allow a bad actor to easily insert liquid drugs into the unsealed port with a syringe.
“Evidence presented at trial showed that Ortiz surreptitiously injected IV bags of saline with epinephrine, bupivacaine and other drugs, placed them into a warming bin at the facility, and waited for them to be used in colleagues’ surgeries, knowing their patients would experience dangerous complications,” the Department of Justice said in a statement about the case.
“Surveillance video introduced into evidence showed Ortiz repeatedly retrieving IV bags from the warming bin and replacing them shortly thereafter, not long before the bags were carried into operating rooms where patients experienced complications. Video also showed Ortiz mixing vials of medication and watching as victims were wheeled out by emergency responders.”
In September 2024, Ortiz was sentenced to 190 years in prison for his actions that led to the death of Kasper and the serious bodily injury of ten patients.
Despite this shocking incident, Baxter, the largest producer of IV bags in the United States, has not changed the packaging design on its IV bags, and no new regulatory safety guidelines have been issued, leaving patients just as vulnerable to a tampering assault today as they were three years ago.
“This could have been avoided with a tamper-resistant design,” said attorney Bruce Steckler, who has filed a lawsuit against the manufacturer on behalf of Kaspar and her husband John, per CBS News. “You can inject any kind of medication into that port without any sign of entry. That’s exactly what happened here.”
Other victims of the IV tampering incident are speaking out in support of the lawsuit and calling for the manufacturer to add enhanced tampering safeguards to its product. Dovi Alderstein, the father of the 18-year-old patient who suffered the near-fatal cardiac event after receiving a tainted IV, said that he believes a simple foil seal could have thwarted Ortiz’s scheme.
“It would have prevented it entirely,” Alderstein said, per CBS. “Jack wouldn’t have gone through that. None of the victims would have. And most importantly, Dr. Kaspar would still be with us.”
While Baxter International has declined to comment publicly on the pending litigation, the company claimed in court documents that the death of Kasper was solely due to the criminal actions of Ortiz, and the company’s IV bags are “in compliance with all applicable codes, standards, regulations, and … laws,” CBS News reported.